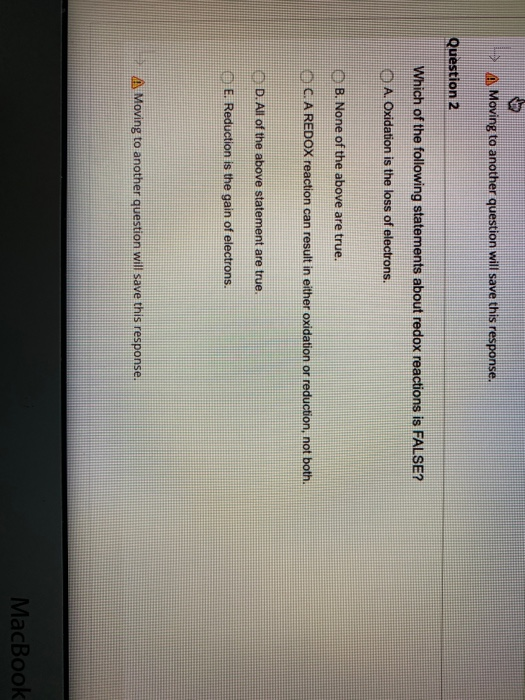
Which of the Following Statements About Redox Reactions Is False
A hydrogen atom is. A The first reaction is a redox reaction.

Ionic Radius Scaffolded Notes Ionic Radius Scaffolded Notes Ionic
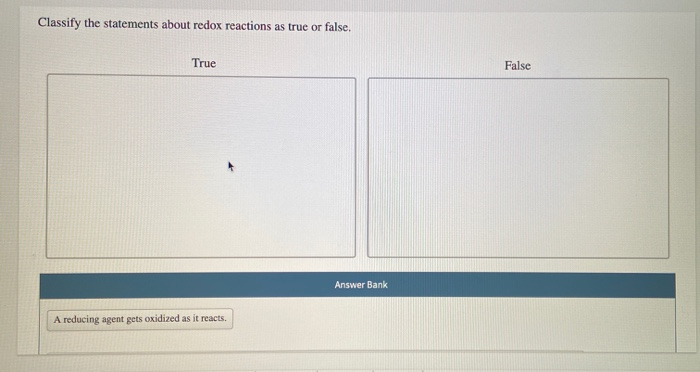
Determine whether the following statement is true or false.
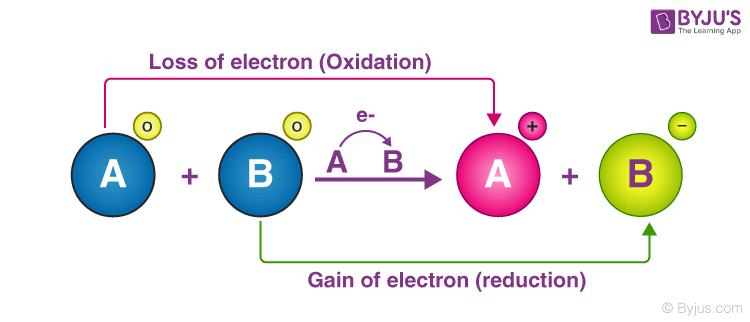
. O The Ecell is positive. Describe the loss and gain of electrons respectively d. 1652 students attemted this question.
H 2 S O d i l Z n C O 3 Z n 2 C O 2 S O 4 2 H 2 O. Which of the following statements about redox potential is false. D A reaction involving elemental oxygen is a redox reaction.
A Oxidation is the loss of electrons. B Reduction is the gain of electrons. Which of the following statements is not true of most cellular redox reactions.
Simple interest earns an interest of 17982. OThe oxidation occurs at the anode. C Addition of the filtrate to starch solution gives blue colour.
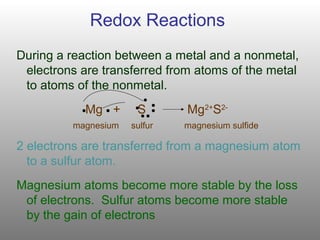
An oxidation-reduction redox reaction is a type of chemical reaction that involves a transfer of electrons between two species. OxygenH2O redox pair has the highest redox potential. Oxidation is the loss of electrons.
B Reduction is the gain of electrons. Both always happens at the same time hence the name redox red-ox reduction-oxidation. Which of the following statements about redox reactions in biochemistry is FALSE.
B Reduction is the gain of electrons. A Oxidation is the loss of electrons. E All of the above statement are true.
Which of the following statements about redox reactions is FALSE. State whether the following statements are true or false and correct the false statement. D A reaction involving elemental oxygen is a redox reaction.
The reactant that is oxidized loses electrons. A O 2 is the strongest oxidant commonly encountered in biochemical processes. C FAD is a stronger oxidizing agent than NAD.
Very Important Questions A cummulative deposit account of monthly instalment of 3600 at 9 pa. 29 what type of reaction is the generic equation A B AB. Thus only option B is incorrect whereas options AC and D are correct.
Cannot occur independently of each other b. The components of the electron transport chain are organized in terms of their redox potential. Biochemistry Biological Oxidation Reduction Reactions.
D A reaction involving elemental oxygen is a redox reaction. SCIA REDOX reaction can result in either oxidation or reduction not both D. OxygenH 2 O redox pair has the highest redox potential.
Electricity must be applied to force the non-spontaneous reaction to occur. Result in a change in the oxidation states of the species involved e. The reaction in the atomic reactor is a type of uncontrolled chain reaction.
Thus it is a redox reaction. Accompany all chemical changes c. Which of the following statements is FALSE for an electrolytic cell.
Energy can be harvested from the glucose molecule and generate ATP only if the cell can carry oxidation-reduction reactions. Changes in potential energy can be released as heat. C A reaction can result in either oxidation or reduction not both.
B is false When a reaction undergoes an oxidationreduction reaction one product was oxidized the other is reduced. Oxidation is loss of electrons and reduction is gain of electrons. Which of the following statements about redox reactions is false.
Reduction is the gain of electrons. Question 2 Which of the following statements about redox reactions is FALSE. The electron acceptor is reduced.
10 Which of the following statements about redox reactions is FALSE. E All of the above statement are truc. Which scientist is credited with early quantitative data on redox reactions.
None of the above are true. Biochemistry Multiple Choice Questions on Biological Oxidation-Reduction Reactions. Which of the following statements isare correct about the following reactions.
Oxidation and reduction a. C 6 H 1 2 O 6 H 2 S O 4 c o n c 6 C 6 H 2 O. NADHNAD redox pair has the least redox potential.
NADHNAD redox pair has the least redox potential. Which of the following statements is are true. C A reaction can result in either oxidation or reduction not both.
Moving to another question will save this response. Electrons flow from the anode to the cathode within the electrochemical cell. All of the above statement are true.
D The white ppt is soluble in NaOH. B Most biological oxidations involve reactions between an organic substrate and O 2. Which of the following statements about redox reactions is FALSE-Reduction is the gain of electrons-A reaction can result in either oxidation or reduction not both-A reaction involving elemental oxygen is a redox reaction-Oxidation is the loss of electrons-All of the above statement are true.
C A reaction can undergo either oxidation or reduction not both. An oxidation-reduction reaction is any chemical reaction in which the oxidation number of a molecule atom or ion changes by gaining or losing an electron. A Oxidation is the loss of electrons.
H 2 S O 4 d i l Z n Z n 2 H 2 S O 4 2. E All of the above statement are true. Which of the following statements about redox potential is false.

Out Of The Following Redox Reactions I Nh4no3 D N2o 2h2o Ii Nh4no2 D N2 2h2o Iii Pcl5 D Pcl3 Cl2 Disproportionation Is Not Shown In

Solved 28 Identify The Oxidation Reduction Reactions Among Chegg Com

Solved Q16 Classify Each Of The Following Statements As True Or False Oxidation Takes Place At Anode T Er Reduction Takes Place At Cathode T74e Electrolysis Is A Spontaneous Reaction False The Oxidation Number
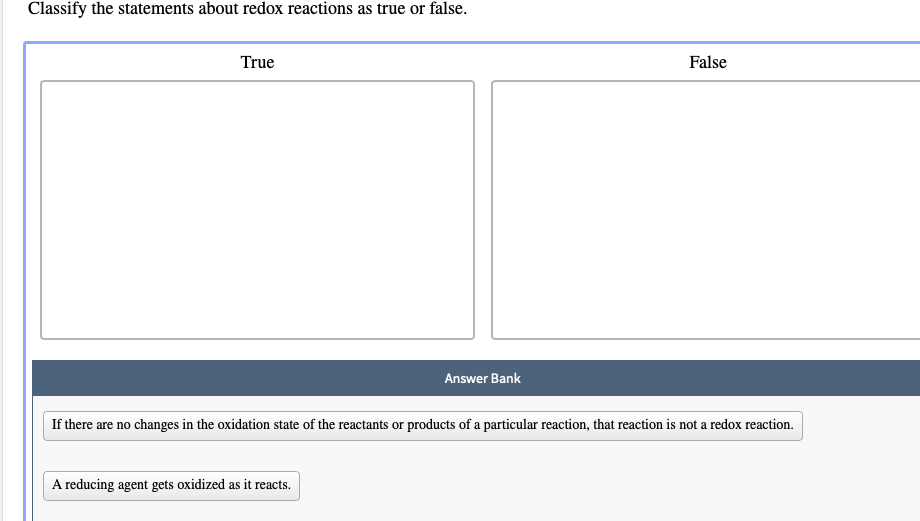
Solved Classify The Statements About Redox Reactions As True Chegg Com

Types Of Reactions Classwork Homework By Threefourthsme Tpt Chemical Reactions Covalent Bonding Chemical Equation

Is Cellular Respiration An Oxidation Or Reduction Reaction
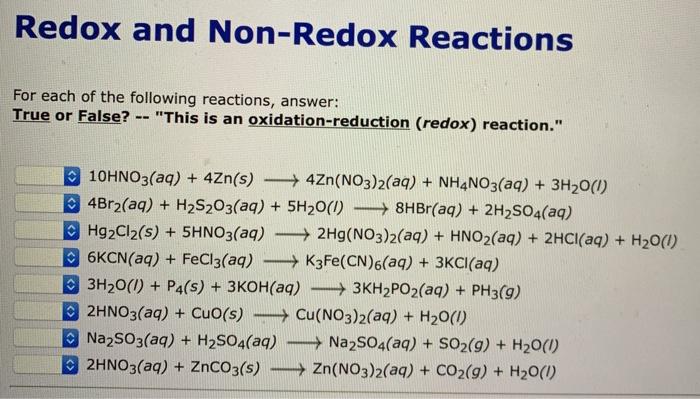
Solved Redox And Non Redox Reactions For Each Of The Chegg Com
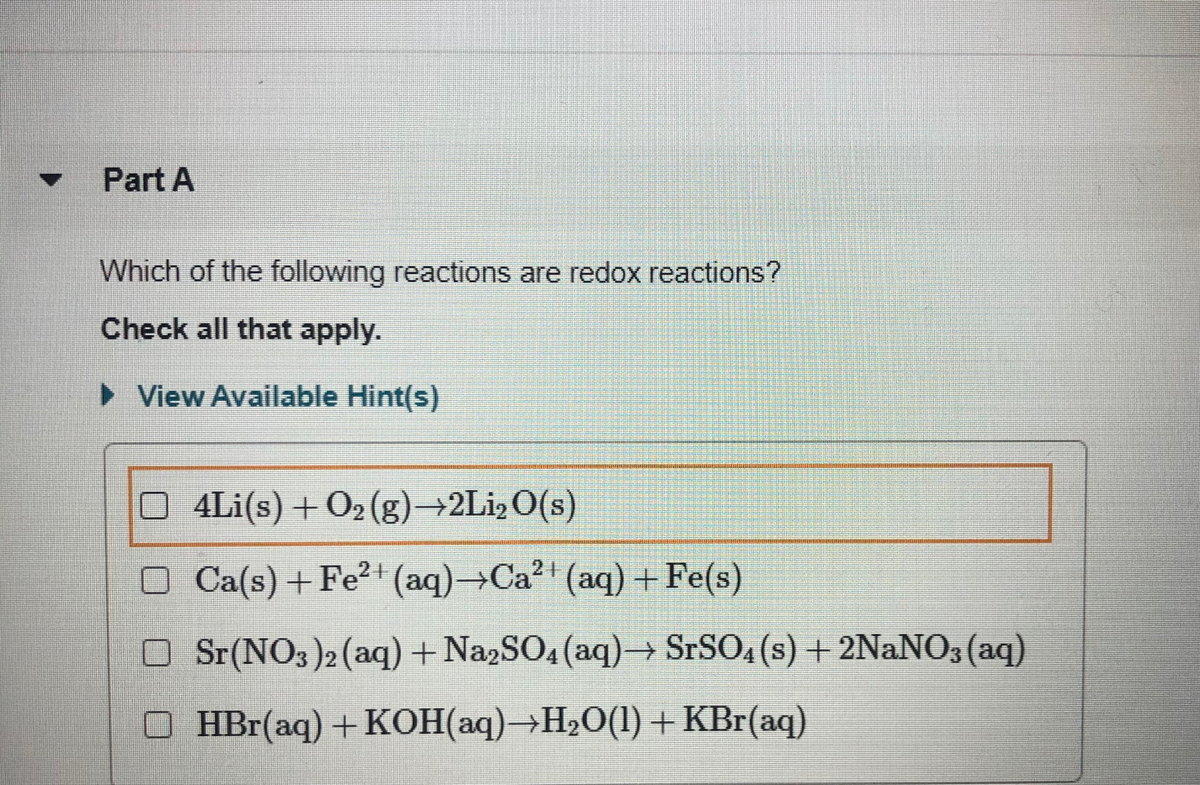
Answered Which Of The Following Reactions Are Bartleby

Pin By Offi Rahman On Icse Board Inbox Screenshot Pandora Pandora Screenshot

Boardworks Gcse Additional Science Chemistry Chemical Reactions Chemical Reactions Additional Science Redox Reactions

Stoichiometry Scaffolded Notes By Threefourthsme Tpt Dimensional Analysis Guided Notes Scaffolded Notes
Solved Moving To Another Question Will Save This Response Chegg Com

Splitting A Redox Reaction Into Half Reactions Oxidation Half Reaction And Reduction Half Reaction Redox Reactions Oxidation Data Science

Solved Classify The Statements About Redox Reactions As True Chegg Com
Solved Classify The Statements About Redox Reactions As True Chegg Com

Learn About Redox Reactions Chegg Com

Assertion And Reason Both Are Correct Statements And Reason Is Correct Explanation For Assertion

Comments
Post a Comment